Due to time constraints, medical research experiments using cells may occur weeks, months or years apart from their extraction. It would be unreasonable to keep growing cells for that duration and instead cell growth is paused. This process, called cryopreservation, freezes tissues to levels where biological activity is effectively paused, allowing for indefinite storage of cells.
Freezing of cells is commonly performed in a liquid suspension with a cryoprotectant, but advances in tissue engineering have enabled the growth of cells in 3D scaffolds called hydrogels, from which cells are difficult to extract and cryopreserve. This has led to the freezing of cells within a hydrogel. Now, researchers from the University of Trento in Italy have assessed the effects of a range of cryoprotectants on cells within a hydrogel (Tissue Engineering Part C: Methods doi: 10.1089/ten.TEC.2017.0258).
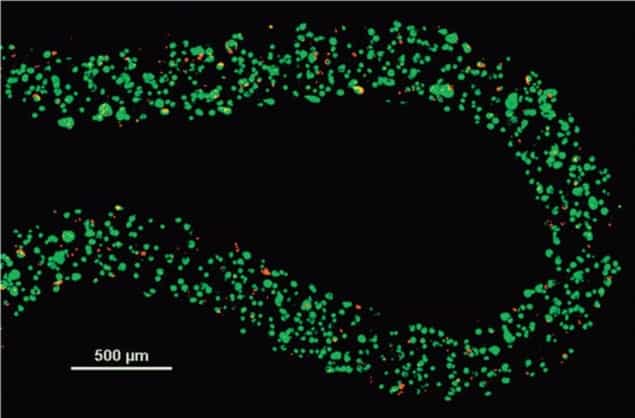
The group found that cells that were encapsulated within the alginate hydrogel had similar levels of cell death to the control (cells within a liquid suspension), but also had higher levels of metabolic and proliferative capabilities when thawed and cultured.
The protocol involved mixing different cryoprotectants – dimethyl sulfoxide (DMSO), glycerol and trehalose – at different concentrations, with cell-laden alginate hydrogels and the control cells. The effect of the different cryoprotectants on the cells within the hydrogel post-thawing had not been investigated previously.
The freezing and thawing of cells is potentially lethal, introducing a lot of stress when not performed under appropriate conditions. Adding cryoprotectants before freezing is crucial as they reduce the freezing temperature of the cell solution, thereby avoiding the damaging formation of intracellular water crystals that can puncture and kill cells. Issues arise when preparing cell solutions for freezing and after thawing as the concentrations used for cryoprotection are toxic to cells when not frozen.
In this study, the researchers identified the best cryoprotectant and optimal conditions for use with the alginate hydrogel: DMSO and glycerol provide the best response in cells after thawing.
Cells used within the lab have a limited lifespan, with only certain amounts of cell doublings before they succumb to senescence or a loss of morphology, highlighting the necessity for safe storage of cells during an extended time-frame. The authors suggest that their approach could be used to evaluate the effects of a range of cryopreservation methods. In addition, they believe the cell encapsulation and cryopreservation techniques could be introduced in tissue engineering as a method for cell banking, cell expansion and for bottom-up development of tissues.



