
In basic and applied biology research, such as investigations of stem cells and drug screening, spheroid cell cultures are valuable, providing a more realistic test environment than the 2D cultures conventionally used. However, existing techniques for making cell spheroids lack reproducibility, have limited production yields and performance can vary with cell type.
Tackling this, researchers in Korea have developed a technique that generates high yields of spheroid cultures with reproducible shapes and sizes using “lab-on-a-CD” technology, also known as centrifugal microfluidics. The approach involves pumping cells outwards from the centre of a small disc into wells where they aggregate into spheroids. The researchers also used the technique to generate co-cultures containing two cell types in specific arrangements for the first time (Biofabrication 9 045006).

“The rapid, reproducible formation of spheroids whose size we can control can contribute to accelerate biology and pharmacy research, especially cancer drug discovery,” said co-author Jung Chan Lee, who carried out the work with colleagues at Seoul National University and collaborators at Chung-Ang University, also in Seoul.
Spheroid creation
Cell spheroid manufacture is the first application of the well-established lab-on-a-CD technology in cell culture research. Already used for a variety of biomedical diagnostic tests including blood analysis and immunoassays, instrumentation is compact, relatively inexpensive and easy to use.
The new system comprises a 6-cm moulded silicone polymer disk on top of a rotating platform driven by a DC motor. The disk contains 100 radial channels measuring 1.4 x 1.5 x 16 mm, each of which feeds a cylindrical microwell 0.4 mm in diameter and 0.4 mm high.
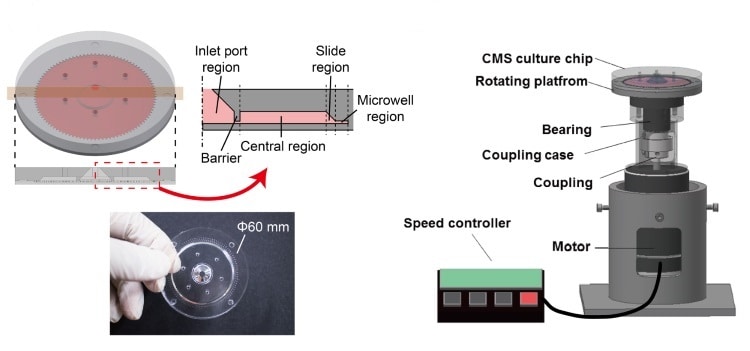
Suspensions of cells are injected through a port in the disk’s centre. Rotatable over a range of speeds, the platform subjects the cells to hypergravity with G-forces of 1-521 G. The centrifugal forces not only drive the cells into the wells and aggregate them into spheroids, but can also encourage cell proliferation.
Lee and co-authors used their system to create cell mono-cultures and co-cultures. They created batches of 100 spheroids at a time, measuring their shape and size using time lapse photography and fluorescence images. Cultures of human adipose-derived stem cells (hASC) and human lung fibroblasts (MRC-5) were created using the microfluidic approach and compared with those generated using conventional well plate-based spheroid (WPS) formation.
Overall, the new system performed better than the conventional method. It demonstrated, for example, a 100% production yield, meaning that significantly fewer cells are needed to generate a given number of spheroids.

Spheroid size was also significantly more consistent using the new technique, but only in spheroids generated at a higher 103 G-Force, suggesting that hypergravity is an important factor. Up to two days following formation, the spheroids were also more uniform in shape than those produced using the conventional method, indicated by smaller standard deviations in sphericity across the batch.
The researchers also successfully generated co-cultures with three spheroid arrangements: a spheroid of stem cells surrounded by a concentric shell of lung fibroblasts, a “Janus” arrangement of two hemispheroids and a sandwich of alternating cell layers. Each arrangement was created by the sequential addition of the different components to the disk at specific time intervals.
The concentric arrangement, for example, was made by injecting a suspension of one cell component, followed by the second three minutes later. At this point, centrifugal forces have driven the first set of cells into a U-shape. The second set of cells settles in this U for the outer layer to close around it.
In ongoing work, the researchers are seeking to further miniaturize their system, said joint first author Jiheum Park of Yale University, who worked on the system at Seoul National University. “One effort is to combine the system with wireless power transfer technology and eliminate the power line, which could be the possible source of incubator contamination,” Park told medicalphysicsweb. The researchers are also investigating the stacking of multiple disks to increase the throughput of the system by up to 100-fold.



