In clinical practice today, proton therapy is planned assuming a constant value of 1.1 for the relative biological effectiveness (RBE) of protons. RBE, however, is a complex variable that depends on many factors and – despite extensive research – there is no accepted variable RBE model available for use in clinical treatment planning. As such, the biological uncertainties of protons remain a major challenge for realizing the full potential of proton therapy.
One factor that affects RBE is the linear energy transfer (LET), with higher LET correlating to increased RBE. Thus it may be possible to modify the delivered RBE by varying the LET instead. With this aim, researchers from the MD Anderson Cancer Center have studied the impact of incorporating LET criteria directly into intensity-modulated proton therapy (IMPT) planning. Their goal: to increase LET in target volumes while reducing LET in critical structures compared with a conventional plan (Phys. Med. Biol. 63 015013).
“Currently, IMPT treatment planning does not evaluate LET distributions specifically; however, LET is an important contributing factor to protons’ biological effectiveness besides physical dose,” explained first author Wenhua Cao. “A conventionally optimized IMPT plan could result in placing protons with high LETs in normal tissues instead of target volumes. This can be improved by LET optimization.”
Replanning results
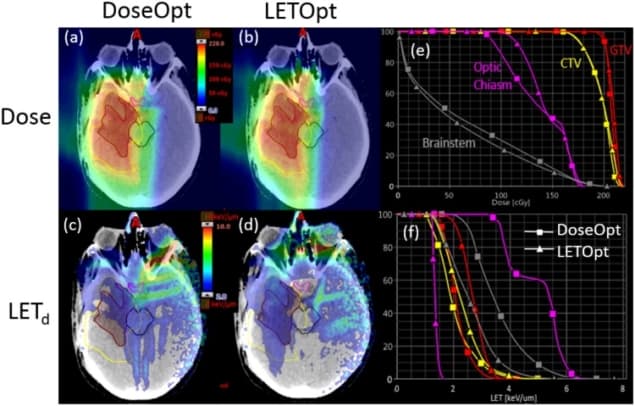
Cao and colleagues examined five brain tumour patients who had been treated with proton therapy. All patients had one or more critical structures adjacent to or overlapping with target volumes. They created two IMPT plans for each patient, one using conventional dose-based optimization (DoseOpt) and the other using the proposed LET-incorporated optimization (LETOpt). All plans had the same target prescriptions and field arrangements as in the clinical treatments, and were tailored to produce similar dose distributions to those delivered clinically.
For each plan, the researchers determined a series of key indices for dose and dose-averaged LET (hereafter referred to as LET): dose and LET for 1% and 99% of the gross tumour volume (GTV); the maximum dose and LET for the brainstem; dose and LET exceeded in 0.1 cc of the brainstem; and maximum and mean dose and LET for the optic chiasm.
For those selected dose indices, they found only minor differences between the DoseOpt and LETOpt plans. The LET indices, however, showed pronounced differences. Maximum LET and LET to 0.1 cc of the brainstem were reduced by an average of 19.4% and 23.7%, respectively, from DoseOpt to LETOpt. The maximum and mean LETs for the optic chiasm, meanwhile, were reduced by 21.1% and 21.9%, respectively, while LETs for 1% and 99% of the GTV were increased by 27.2% and 18.4%.
Looking at an example glioblastoma case, dose distributions and dose–volume histograms confirmed that the doses generated by the two plans were comparable. LET distributions and LET–volume histograms verified that sparing of the brainstem and optic chiasm were significantly improved in the LETOpt plans. The researchers note that the LET increase in the GTV was less pronounced than the LET decrease in the organs-at-risk.
In another patient (an ependymoma case), the two plans again delivered similar doses, although the DoseOpt plan produced worse brainstem sparing in the low-dose region than the LETOpt plan. LETOpt greatly reduced LET hotspots in normal tissues and the brainstem, and produced plans with a larger high-LET area in the target than the DoseOpt plans.
For this patient, the researchers also examined how the ratio of optimization priority factors of the dose and LET objectives impacted the final plan. They created two LETOpt plans with this ratio set as one (plan 1) or 10 (plan 2). For plan 1, although the brainstem was not well spared at low doses by LETOpt, its exposure to high LETs was greatly reduced. In plan 2, dose sparing of the brainstem was similar for LETOpt and DoseOpt, but the benefit of LET sparing could not be achieved as it was in plan 1.

While both LETOpt plans increased LET in target volumes, the size of this increase was less for plan 2 because higher optimization priority was given to dose. The authors suggest that, in the clinic, the choice between plan 1 and 2 should be determined by the physician’s preference with regard to different metrics (such as dose to the brainstem or boost in target dose, for example).
The results demonstrated that LET-incorporated IMPT optimization can increase dose-averaged LET in target volumes and reduce it in critical normal tissues, while maintaining satisfactory dose distributions. The team point out that clinical application of this method requires no changes to the current treatment protocols, and could be implemented immediately to benefit patients.
“We have treated only one patient so far with LET-optimized IMPT at our centre, but plan to treat an increasing number,” Cao told medicalphysicsweb. ” We are working to set up a protocol in order to use LET optimization systematically for IMPT. We believe the LET-optimized IMPT will benefit patients with high risk of radiation-induced toxicity for critical structures close to tumour targets.”



