Increasingly, radiation oncology clinics are favouring small, mobile kilovoltage X-ray tubes over radioactive sources to deliver intra-operative radiotherapy. The devices are used to irradiate surgical cavities following the excision of tumours from sites such as the breast. Short-range radiation minimizes the exposure of nearby healthy tissue, enabling large doses to be delivered in a single shot.
Introduced to clinical practice within the last 20 years, the dosimetry of such so-called electronic brachytherapy sources is still under development. Currently, manufacturers typically use their own protocols, making comparisons of doses delivered by different devices problematic.
“Having an accurate methodology for independently verifying the dosimetry of these devices is crucial for performing dose comparison studies, or for planning treatments that combine miniature X-ray sources with other radiation modalities,” says Peter Watson, a PhD candidate at McGill University in Montréal, Canada.
In a new study, Watson and co-authors Marija Popovic and Jan Seuntjens developed a formalism for the absolute dosimetry of therapeutic kilovoltage sources, applying it to the INTRABEAM system manufactured by Carl Zeiss Meditec (Phys. Med. Biol. 63 015016).
Calibration check
The INTRABEAM system produces an isotropic distribution of 50 kVp bremsstrahlung and characteristic X-rays using a gold target. The manufacturers provide a factory calibration that enables reproducible doses to be delivered for treatments, correcting for fluctuations in output using daily quality assurance (QA) measurements. System output is also checked annually by the manufacturers.
“This dose is ‘correct’ in that it has been shown to be empirically safe and effective in treatments,” explained Watson. “However, exactly how closely this represents the actual physical dose, by definition of absorbed dose to water, is an open question.”
As an optional QA tool, dose delivered by the INTRABEAM system can be measured using a manufacturer-supplied water phantom and a thin window parallel plate ionization chamber. A depth-dependent correction function supplied by the system manufacturer enables comparison of the doses with system calibration data.
However, information on the correction function is “limited” and how or if the factory measurements or those using the water phantom are traceable to an absorbed dose standard is unclear. Consequently, Watson and his co-authors set out to assess doses as measured by the QA tool against their formalism.
In the new approach, absolute dose in water in the clinical beam is calculated using CQ, a depth-dependant ionization chamber conversion factor. The factor converts the chamber air kerma calibration coefficient, measured in a reference beam, to an absorbed dose to water coefficient for the INTRABEAM photon spectrum in water.
To calculate CQ, the researchers developed a Monte Carlo model of the INTRABEAM source and parallel plate chamber based on the EGSnrc particle transport code. By using direct calculations, the approach does not rely on cavity theory and its associated assumptions. The model was validated by comparing the half value layers (HVL) and depth doses it calculated with measurements, revealing good agreement between the two.
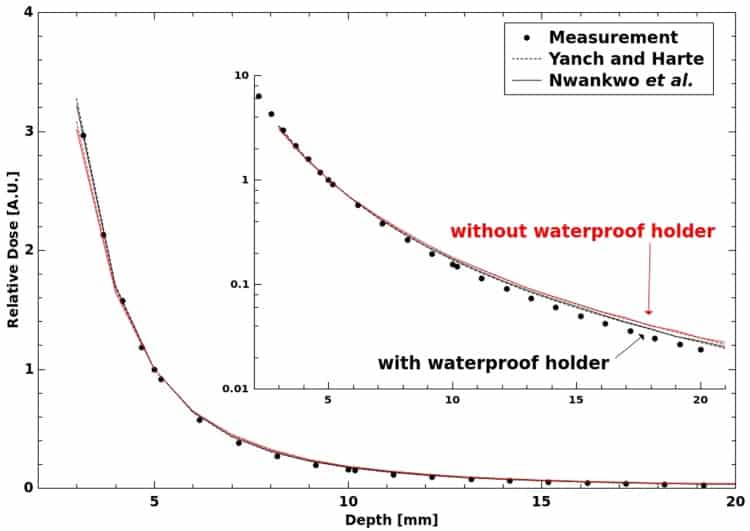
The simulations did, however, reveal significant differences between CQ and the equivalent parameter reported by the manufacturer, corresponding to discrepancies in absolute dose of up to 23%. The model consistently produced a greater CQ value. Differences were seen across the quoted tolerance in chamber plate separation and two corresponding effective points of measurement, as well as over a range of depths. Uncertainty in the plate separation alone accounted for variations in CQ of up to 15%. The results highlight a need for investigations using another chamber with more precisely known dimensions, Watson told medicalphysicsweb.
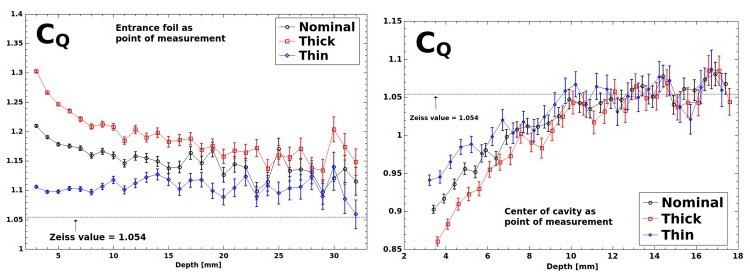
In ongoing work, the authors have compared dose calculations using the formalism with the INTRABEAM manufacturer’s QA approach and prescribed doses derived from the factory calibration, as well as measurements using GafChromic film.
Research such as the ongoing TARGIT-B breast cancer trial could benefit from the greater certainty in doses provided by the formalism. In the multi-centre trial, researchers are comparing cancer control provided by whole-breast external-beam radiotherapy combined with either intra-operative or external beam boosts to the tumour bed.