
Proton therapy plans rely on estimates of particle range in the patient, typically derived from X-ray CT scans, with the CT numbers converted into proton stopping-power ratios (SPRs) using a generic Hounsfield look-up table (HLUT). But this approach cannot account for differences in tissue composition, or patient-to-patient variations, thus limiting the treatment accuracy. To manage range uncertainties arising from this CT-to-SPR conversion, it was suggested back in 1985 that a safety margin of 3.5% of the total range should be applied for all proton treatments.
Speaking at the recent ESTRO 37 congress, Christian Richter from Helmholtz-Zentrum Dresden-Rossendorf and OncoRay pointed out that in 2018, the majority of proton centres still employ this 3.5% range uncertainty margin. “We currently do not use the full potential of this advanced technology. Provocatively speaking, it’s like driving an aeroplane rather than flying it,” he told the delegates.
We can do better, Richter explained, by using dual-energy CT (DECT) to determine particle range. DECT uses two scans with different X-ray spectra to provide complementary information on tissue composition. But although DECT has been clinically available in radiology for about a decade, its use in particle therapy is far from being a clinical standard.
“At the German National Center for Radiation Research in Oncology (NCRO), partnered by OncoRay in Dresden and DKFZ in Heidelberg, we have set up a project to focus on the translation of DECT into particle therapy,” said Richter. The goal is to demonstrate and quantify the benefit of DECT-based patient individual range prediction (PIRP).
In a first step, the NCRO team validated PIRP in extensive high-accuracy ground-truth settings, in both biological tissue and realistic anthropomorphic geometries. These studies demonstrated the superior accuracy of PIRP/DECT over the HLUT approach. “For example, Christian Möhler from the NCRO team proved that PIRP accuracy in multiple biological tissues was better than 0.2%,” Richter noted.
And since April 2015, OncoRay has been using DECT, along with state-of-the-art HLUT, to calculate treatment plans for the majority of patients in its proton therapy facility in Dresden. This approach provides improved image quality and flexibility for proton planning. And with over 2500 DECT scans in their database now, it also acts as a great research resource.
Reducing the uncertainty
To investigate uncertainty levels in more detail, Richter and colleagues have studied intra- and inter-patient variabilities in CT-to-SPR conversions. In over 100 brain tumour patient, for example, they observed an average 4.5% intra-patient variation in the relationship between SPR and CT number in soft tissue. Such variation is intrinsically considered by the PIRP approach, he noted.
Another study of over 100 head tumour patients revealed that conversion factors also differ according to the patient’s age. In bony tissue, there was a 5% difference in the relationship between SPR and CT number between adults and children, due to differing calcium content in the bone. “This variation is not covered by the one-fits-it-all HLUT approach,” said Richter.
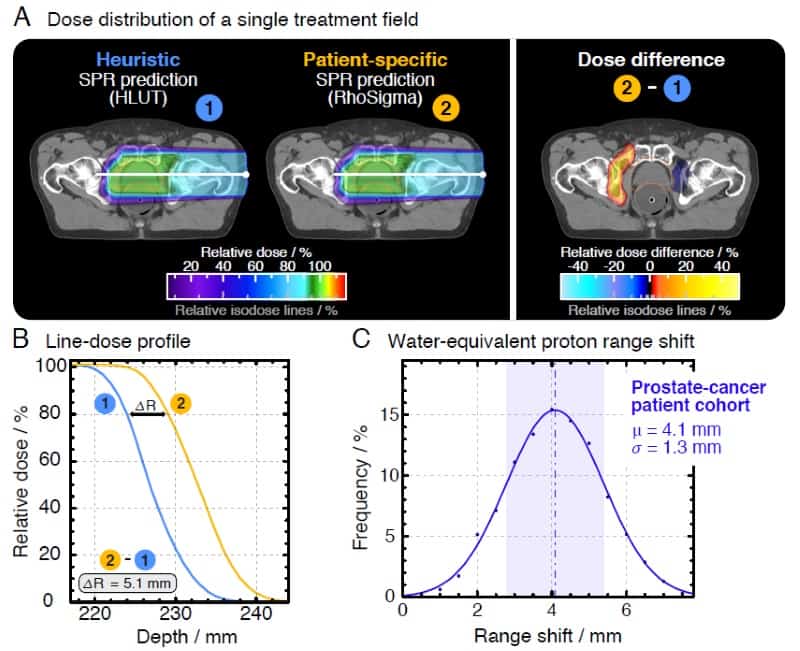
The team, led by OncoRay’s Patrick Wohlfahrt, performed a study assessing range differences between CT-to-SPR conversions using HLUT and PIRP, in 25 brain tumour and 25 prostate cancer patients. In the latter group, they observed average range deviations of 4.1 mm (1.7%) for HLUT compared with the more accurate DECT. “This is clinically relevant, this does matter,” Richter emphasized. He added that one approach is to adapt the look-up-table to better match the PIRP result. “This DECT-based HLUT refinement is an important step – for the first time, PIRP has influenced clinical range prediction,” he said.
One potential stumbling block in this technique is that DECT scans are acquired consecutively, and that motion in and between scans may perhaps affect reliability. Richter described a proof-of-principle study analysing the clinical feasibility of using dual-spiral 4D-DECT scans for proton dose calculation in three non-small cell lung cancer patients. No anatomical differences were seen in the 4D-DECT data processed from consecutively acquired scans, validating the feasibility of PIRP.
The higher imaging accuracy afforded by DECT, as well as its intrinsic consideration of patient variability, will ultimately reduce uncertainty in range prediction for particle therapy. DECT also benefits applications such as identifying and quantifying metallic implants, or providing improved inputs for Monte Carlo calculations. “I see only advantages without major drawbacks,” said Richter. “So why don’t we use DECT more routinely in radiation oncology?”
The NCRO team is now working to achieve this goal, collaborating with a manufacturer to develop a prototype DECT system for clinical implementation and creating a dedicated imaging protocol for proton therapy scans. “I think this is a game changer – after 30 years we should finally be able to reduce the margins,” Richter concluded.



