In a proof-of-concept study, American researchers have used CRISPR/Cas9 genome editing to reverse diseases such as diabetes and muscular dystrophy in mice. For the first time in an animal model of a human disease, this approach has been used to activate genes instead of breaking the DNA structure, alleviating a previous cause of major concern for researchers (Cell 171 1495).
Over the past few years, researchers have intensively explored the potential of the CRISPR/Cas9 method to modify DNA by deleting faulty sequences. In short, the scissor-like enzyme Cas9 is coupled with a guide RNA molecule that binds it to a specific location on the DNA structure. Once attached to the DNA, the Cas9 enzyme breaks the two strands of the DNA helix, which causes deletion of the targeted sequence. Recently, however, concerns were expressed over the possibility that the enzyme may eliminate other parts of the DNA strands, creating unwanted damage.
Boosting gene expression…
A team led by Juan Carlos Izpisua Belmonte from the Salk Institute in the US decided to use an alternative approach. They focussed on influencing the gene activity without altering the DNA sequence: instead of cutting out faulty genes, they tried to counterbalance the genes’ effects by activating antagonist ones.
To achieve their aim, the researchers designed a novel technique that combines the Cas9 enzyme with a shorter guide DNA – 14 to 15 nucleotides rather than the 20 used in most CRISPR/Cas9 techniques – thereby preventing Cas9 from cutting. The enzyme is coupled with a transcriptional activation domain that turns on targeted genes and boosts their activity.
The Cas9/RNA complex is usually carried to the DNA site-of-interest by an adeno-associated virus (AAV), but the protein created by fusing Cas9 and the activation domain proved too large for such a vehicle. The load was consequently divided and charged into two separate AAVs: one carrying the Cas9 enzyme and the other transporting the activator and shorted RNA.
…To restore impaired abilities
To prove the efficiency of their new method, the researchers tried to boost the expression of specific genes linked to impaired functions in diseased mice.
For example, they targeted the genes responsible for the production of insulin in a group of 16 diabetic mice to restore the animals’ ability to control blood sugar. When compared with a control group of 12 non-treated diabetic mice, the level of blood glucose was significantly lower in treated mice.
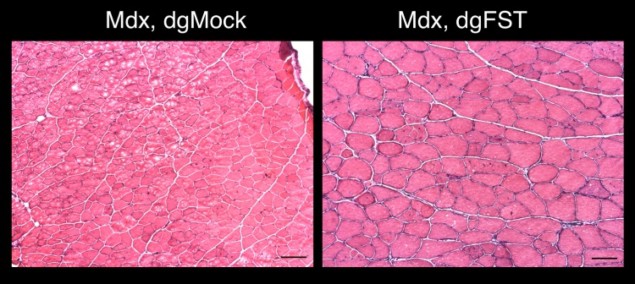
Similarly, Belmonte and his team also demonstrated that their technique could activate previously impaired or silenced genes to restore normal kidney function in acute kidney injuries, or recover muscle growth in a mouse model of muscular dystrophy.
In practice soon?
This original approach differentiates itself from alternatives by leaving the gene mutation unaltered, but instead recovering the expression of other genes in the same pathway. Preliminary data suggest that the technique is safe and does not trigger any unwanted genetic mutation, but further studies are required to ensure that it can be safely brought in clinical practice.



