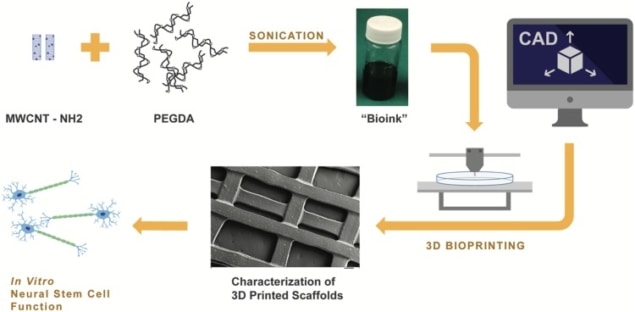
In the search for therapies that repair nerves damaged through injury or disease, researchers are increasingly looking to 3D nerve scaffolds as an alternative to stem cell transplants. Seeded with neural stem cells, scaffolds can mimic the extracellular architecture of neural tissue physically and biologically, creating an environment that promotes cell proliferation and differentiation and therefore nerve repair.
In a new study, researchers in the US used 3D printing to fabricate a porous nerve scaffold incorporating electrically conducting multi-walled carbon nanotubes (MWCNT). The nanotubes produced greater neural stem cell proliferation and differentiation. Differentiation increased further still when small currents were applied to the scaffold, artificially stimulating action potentials in the seeded cells and acting as an electrical cue (J. Neural Eng. 15 016018).
“Our research demonstrated that customizable 3D printed MWCNT scaffolds are excellent for promoting neural differentiation and are thus promising candidates for future neural regeneration applications,” said first author Se-Jun Lee, a PhD candidate at the George Washington University in Washington DC.
Lee and colleagues used a bioink containing a 60% poly(ethylene glycol) diacrylate (PEGDA) solution and varying concentrations of nanotubes to fabricate the scaffolds. Upon exposure to a 355-nm ultraviolet laser during printing, a photoinitiator in the bioink acts as a curing agent, polymerizing the PEGDA and transforming it into an insoluble and biologically inert hydrogel. The use of 3D printing enabled control of the size of the scaffold’s pores, a parameter previously reported to influence cell behaviour.
The nanotubes measured 1–1000 nm in length and 15–37 nm in diameter, and were amine functionalized. Addition of amine groups enables dispersion of the nanotubes in the bioink; in a pure form the nanotubes clump. Research in the literature has also reported that amines can promote neuron growth.
Incorporation of the nanotubes produces a scaffold surface that mimics that of neural tissue on a nanoscale and to which neurons can adhere, as well as promoting proliferation and differentiation. Their high conductivity enables electrical stimulation of the scaffold. In turn, the stimulus induces action potentials in cells on the scaffold that act as an electrical cue for proliferation and differentiation.
Scaffold characterization
In a series of tests, Lee, supervisor Lijie Grace Zhang and co-authors characterized the resulting scaffolds seeded with mouse neural stem cells to assess their efficacy. They quantified cell proliferation through an Alamar Blue assay and absorbance measurements using a spectrophotometer, using 10-mm diameter scaffold samples each seeded with 30,000 stem cells. At seven days following culture, the test revealed significantly greater proliferation in scaffolds with the highest nanotube concentration compared to control scaffolds with no nanotubes.

The researchers also measured early stage cell differentiation on the scaffold and the outgrowth of neurites – axon precursors, using fluorescent staining combined with laser scanning confocal microscopy. Both showed increases upon incorporation of nanotubes. For example, the total neurite length in scaffolds with the highest nanotube concentration, 0.1%, was 113% greater than in scaffolds with no nanotubes. The same images also illustrated attachment of stem cells to the scaffolds.

Electrical stimulation increased cell differentiation further still. In tests four days following culture, scaffolds subjected to a pulsed current of 500 A expressed significantly higher levels of the TUJ1 protein, the neuromarker used by the researchers to detect early differentiation, than non-stimulated controls.
Based on their findings, the authors are optimistic that scaffolds such as theirs could be used clinically in the future. In ongoing work, they are investigating the potential of electrically stimulated MWCNT to transform mesenchymal stem cells into Schwann cells, a type of glial cell.
“Schwann cells play a critical role in nerve repair by myelinating the axons of the peripheral nervous system,” Lee told medicalphysicsweb. “If successful, our study will further demonstrate that 3D printed MWCNT scaffold can aid in peripheral nerve damage repair.”



