
The onset of many neurodegenerative diseases, such as Alzheimer’s and stroke, is directly related to the dysfunction of the neurovascular unit (NVU). For this reason, 3D models that closely mimic the complexity of the NVU are needed to investigate what goes wrong during pathogenesis. Tissue engineering offers a plethora of advanced techniques to achieve this goal. Among them, bioprinting seems the most promising, allowing the deposition of novel biomaterials and bioinks to recreate NVU-mimicking structures.
A recent review by researchers from The University of Manchester has highlighted the advantages and promising outcomes of using bioprinting in the study of neurodegenerative diseases (Trends in Biotechnology doi: 10.1016/j.tibtech.2018.01.003).
Neurovascular unit components
The NVU is a brain-localized unit, composed of a vascular and a neural component. The correct cross-talk between these two components is fundamental for brain health, since it regulates transport of oxygen and nutrients to the brain, along with removal of toxic compounds, preventing neuroinflammation and neurodegeneration.
The NVU extracellular matrix (ECM) represents the medium through which neural and vascular components can interact, providing physical support and biochemical cues to embedded cells. Indeed, the ECM can provide stimuli to promote growth of new neurons and blood vessels, as well as maintenance of brain health over time. For these reasons, disruption of the NVU has been linked to the onset and progression of severe neurodegenerative diseases, such as Alzheimer’s, vascular dementia, Parkinson’s and stroke.
Modelling the neurovascular unit
To understand what goes wrong during dysfunction of the NVU, researchers need new models that can accurately replicate the complex interactions between cells and ECM components in the NVU. Although different animal models exist for this purpose, there is a need to develop human cell-based models, which can be studied in large scales, to dissect precise cellular roles in disease, and for specific human-related experiments.
In this direction, recent progress in tissue engineering has made possible the fabrication of complex structures consisting of different cell types and different materials. Bioprinting stands out among novel tissue engineering techniques, since it allows the precise deposition of cells and material in a layer-by-layer fashion to recreate organized 3D constructs. Bioprinting can be either direct, when the biological material – bioink – is deposited directly to recreate the 3D structure, or indirect, when final structures are obtained by removing additional material used as temporary mould.
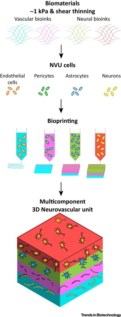
Exploiting these techniques, cells can be encapsulated in water-rich gels, also called hydrogels, which are in turn printed to form 3D structures. For example, endothelial cells can be printed in patterns that mimic the microvasculature of the vascular unit, while neurons and glia can be deposited as interconnected parts to fabricate the neural component. Moreover, these two components can be interfaced by simply printing them as two consecutive layers to mimic the neuro-vascular interactions.
Due to the role played by ECM, researchers can also choose to tune the mechanical and biochemical cues of the NVU models by choosing different bioinks. For example, stiffer or softer hydrogels can be printed according to the stiffness required, while inner porosity and local chemistry of the matrix can be dictated by printing natural or synthetic materials with inherent characteristics.
Therefore, the specific choice of bioinks can lead to fabrication of high-quality and complex NVU models. Moreover, the possibility to finely design these constructs and produce them in large scale makes 3D bioprinted NVU models a valid research tool for the future study of neurodegenerative diseases, moving the field a step forward.



