Hadron therapy shows potential to improve the effectiveness of cancer radiotherapy. By exploiting the characteristic depth-dose distributions of hadrons in tissues (the Bragg peak), the technique offers precise delivery of radiation dose. The large gradients of the highly conformal dose distribution, however, make it critical to precisely and accurately plan the radiation field, align it with the target, and determine the stopping power of the ions. Current methods to do this have an uncertainty on the predicted range of 3–4%.
Advanced methods for absolute range verification are in development throughout the world. Researchers at Heidelberg University Hospital and the German Cancer Research Center (DKFZ) have developed a prototype detection system for helium-beam radiography (αRad) using silicon pixel detectors. The long-term goal of this research project is to develop a range verification method based on αRad.
The team has successfully demonstrated a proof-of-principle of αRAD, based on energy deposition measurements of single ions in thin layers, ion tracking and identification. In one key result, they showed that the rejection of secondary hydrogen ions originating from nuclear interactions distinctly improved the contrast-to-noise ratio (CNR) of the radiographs (Med Phys. 45 817).
Lead author Tim Gehrke and colleagues created a prototype detection system consisting of three parallel silicon pixel detectors. The team selected pixel detectors (the CERN-developed Timepix chip) because they can measure the position of an ion in a single thin layer, and also provide unambiguous position determination in cases where multiple ions hit the detector in the same time window. These capabilities facilitated the construction of the ion track, and result in small multiple Coulomb scattering (MCS) in the tracking system.
The team selected helium ions instead of protons because they reasoned that helium ions would generate a better spatial resolution and undergo less pronounced MCS in the object being imaged. Moreover, they expected to achieve this resolution gain without any disadvantage in terms of CNR or applied radiation dose compared with proton radiography.
Detector verification
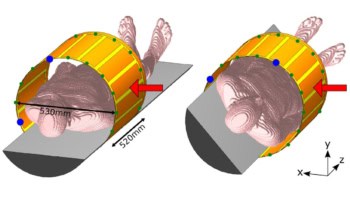
The authors used a phantom comprising a 160 mm thick block of acrylic glass, approximately the size of a human head. The phantom had a mean water-equivalent thickness (WET) of 192 mm and contained maximal WET-variations of ±6 mm. They imaged the phantom with a 173 MeV/u helium ion beam at the Heidelberg Ion-Beam Therapy Center.
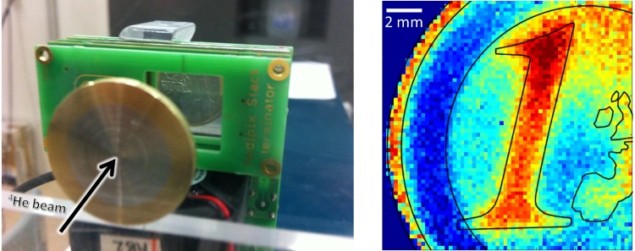
The detection system was placed behind the phantom to register the ions leaving it. The system enabled detection of single particles, so that any with energy deposition higher than a user-defined threshold (in this case 5 keV) could be identified. Three detector layers were deployed: two to measure the arrival time of the ions for tracking and one to measure the energy deposition of the single ions.
When a heavy charged particle impacts the detector, the signal spreads over several adjacent pixels, forming a cluster. The so-called cluster volume, which is the sum of the pixel values in a cluster, was converted into the energy deposition of the incident particle by pixel-wise calibration. The authors note that desirable signals for image formation are clusters caused by single incident ions traversing all three detector layers, creating a matched event. They selected clusters corresponding to helium ions that did not have artefacts or degraded information.
The authors performed data processing, tracking and backprojection of the ions, calculated CNR, estimated the delivered dose and evaluated spatial resolution. They reported that this particular set-up achieved a spatial resolution of at least 1.15 lp/mm for, as well as a resolution of relative thickness differences of 1.2%, at a dose level typical for diagnostic radiography.
Range verification
Helium-beam radiography can be applied as an image-guidance tool for proton, helium and carbon-ion radiotherapy. Gehrke explained that a goal of αRad is to measure WET maps that might be used for in vivo range verification.
“Since the WET measured by αRad retains its validity for proton and carbon-ion beams, αRad is in this respect equally useful for proton, helium and carbon-ion therapy,” he said. “From a practical standpoint, αRad is especially interesting for helium-beam therapy, because this combination of imaging and treatment modality would not require any ion-type switching during the whole process. The applicability of αRad for intra-fractional imaging in proton or carbon-ion treatments will depend on the speed of the ion-type switching of a particular facility.”
The researchers have also recently performed an in-depth comparison of proton and helium-beam radiography, using the silicon pixel detectors and the data processing procedures that they developed. The detection system could be successfully applied to perform αRad, as well as proton radiography (pRad). This research showed theoretically and experimentally that helium ions provide a higher spatial resolution compared with to protons, retaining the same CNR at a comparable dose (Phys. Med. Biol. 63 035037).
Gehrke says that the team is currently focusing on new ideas and concepts to overcome two major limitations of the method. They are working on the extension of the sensitive WET range (currently about 12 mm) in which a high CNR can be achieved. They are also developing methods to decrease the acquisition time of radiographs measured by their prototype detection system, therefore requiring silicon pixel detectors with a higher readout speed.